Background: Mantle cell lymphoma (MCL) is an extremely heterogenous cancer, with substantial variation in clinical presentation and genetic makeup among individual patients. Chronic exposure to cytokines or inflammatory mediators is hypothesized to mediate pathogenesis and influence presentation in MCL, thus representing a key area of interest for prognostication and identification of therapeutic targets. However, measuring a large panel of individual cytokines, binding proteins, and soluble receptors does not give an integrated measure of how much a given pathway is activated within individual cells from a patient. Therefore, we have developed a cell-based reporter assay that can measure in an integrated way the activating potential of patient plasma samples on four central transcription factor-driven pathways: the interferon pathway (STAT1), the acute phase response (STAT3), lymphocyte-targeted cytokines (STAT5), and the inflammatory pathway (NF-κB). In this study, we applied this Integrated Functional Quantitation of Modulation of Transcriptional Pathways (IFQ) assay to plasma from patients with untreated MCL. We hypothesized that the IFQ assay of these transcriptional pathways would be associated with response to treatment and provide prognostic information on the outcome of these patients.
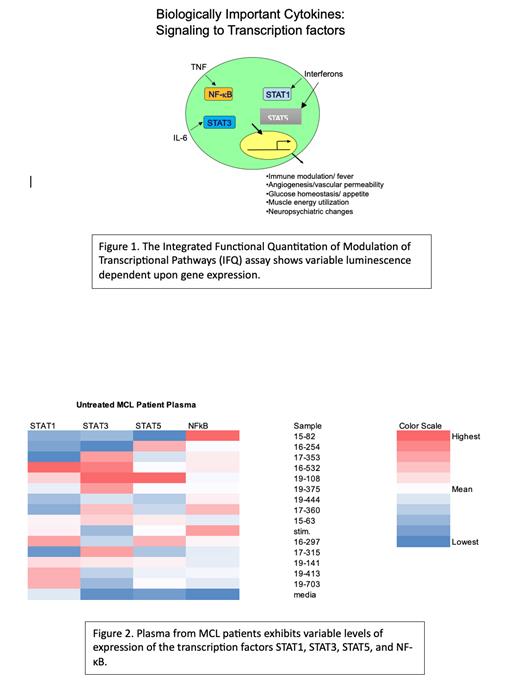
Methods: Reporter cell lines were generated in which the activity of each of these four transcription factors, STAT1, STAT3, STAT5, and NF-κB, regulated expression of a luciferase reporter gene (Figure 1). We identified stored plasma samples collected from patients with untreated MCL who had consented to an IRB-approved research protocol. Cells from the 4 cell lines were plated in quadruplicate in 96-well plates at a concentration of 5000 cells per well and treated with patient plasma prior to incubation for 1 hour. Stimulator media was then added to each well and the plates were incubated for 6 hours after which luciferase activity was quantitated by luminometry. Values were normalized to stimulator-only control and media-only control.
Results: Plasma from 14 patients with untreated MCL was analyzed. The luciferase assay system was successful for all patients, with results shown in Figure 2. Five of 6 patients with downregulation of STAT1 compared to stimulator-only control went into remission without a reported relapse following frontline therapy, whereas both samples with upregulation of STAT1 later had relapsed disease within 1 year of treatment completion. Conversely, 4 of 6 samples found to have upregulation of STAT3 went into sustained remission following therapy, while the single sample showing downregulation went into sustained remission after frontline therapy. Six samples did not reveal a conclusive change in regulation of the STAT3 gene when measured against the control. When evaluating NF-κB expression levels, 12 samples showed downregulation of the gene compared to the stimulator-only control, although no pattern in treatment response was discerned. STAT5 expression showed fewer differences compared to the stimulator-only control group, as 12 of 14 plasma samples conveyed no upregulation or downregulation of the gene.
Discussion: Evaluation of transcription factor expression in the plasma of patients with MCL using the luciferase reporter system is feasible. In our patient population, NF-κB was downregulated in the majority of patients, whereas STAT1 and STAT3 showed differential patterns of expression. STAT1 and STAT3 merit further investigation as possible biomarkers of treatment response, perhaps due to their role in initiating and sustaining inflammation. In the future, we hope to augment these data to strengthen this correlation and to implement this assay to aid in prognostication and treatment selection.
Disclosures
Koff:BeiGene: Consultancy; Viracta Therapeutics: Research Funding. Cohen:Novartis: Research Funding; BMS/Celgene: Research Funding; Genentech: Research Funding; BioInvent: Research Funding; Lam Therapeutics: Research Funding; Takeda,: Research Funding; ADCT: Consultancy; AstraZeneca: Consultancy, Research Funding; Abbvie: Consultancy; Janssen: Consultancy; BeiGene: Consultancy; Loxo/Lilly: Consultancy, Research Funding. Frank:Roche-Genentech: Research Funding; Kymera: Consultancy; ProRavel: Consultancy.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal